Optimising malaria vaccine uptake
OPT-MVAC is a partnership among 14 West and Central African countries pursuing malaria vaccine implementation, and leading African and European research institutions.
Together, we’re supporting implementation research led by national immunisation, malaria and pharmacovigilance programmes to tailor delivery strategies to local needs and promote cross-country learning.

Expanding the malaria prevention toolbox
The availability of malaria vaccines is a historic landmark in malaria prevention that could help save tens of thousands of young lives every year. The World Health Organization currently recommends two malaria vaccines (RTS,S and R21 Matrix/M) to help protect children from the disease in malaria-endemic countries. However, a variety of implementation challenges must be addressed to fully realise this potential.
OPT-MVAC consortium partners are supporting the rollout of malaria vaccines in areas with moderate to high transmission rates. This is being achieved through a vaccine implementation research programme, by adapting delivery approaches to local contexts, and sharing data and best practices across our network.
Children under 5
accounted for 76%
of all malaria deaths in 2023
Africa bears the majority of the malaria burden, recording
94% of global cases
in 2023
Powerful trio:
vaccines, chemoprevention and bed nets
are complimentary and can be used together
14 Countries in West and
Central Africa
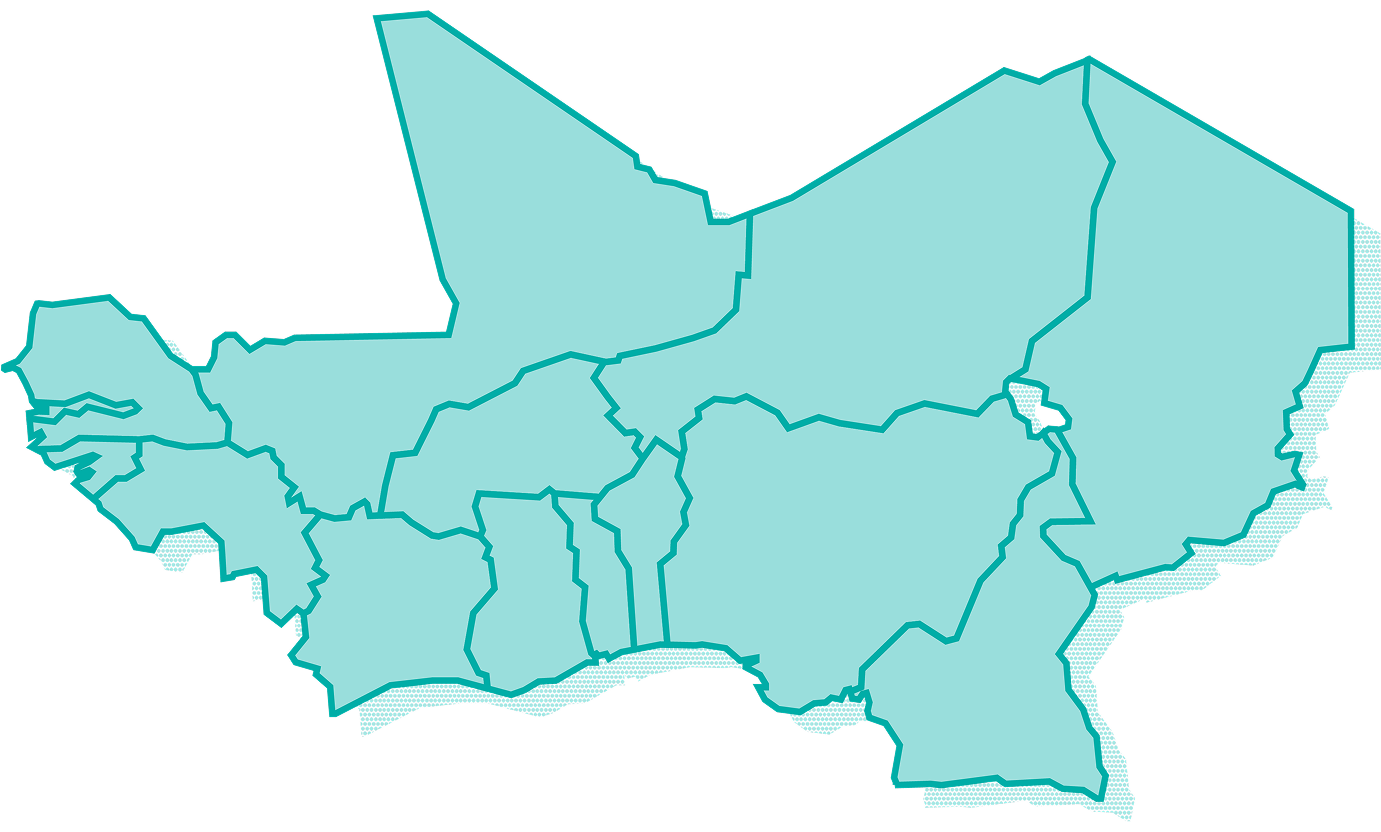
 Benin
Benin
Implementation date:
April 2024Approach:
Age-based
Vaccine type:
RTS,S/AS01
Other malaria prevention tools deployed:
Seasonal malaria chemoprevention, perennial malaria chemoprevention
 Burkina Faso
Burkina Faso
Implementation date:
February 2024Approach:
Age-based
Vaccine type:
RTS,S/AS01 & R21/Matrix-M
Other malaria prevention tools deployed:
Seasonal malaria chemoprevention
 Cameroon
Cameroon
Implementation date:
January 2024Approach:
Age-based
Vaccine type:
RTS,S/AS01
Other malaria prevention tools deployed:
Seasonal malaria chemoprevention, perennial malaria chemoprevention
 Chad
Chad
Implementation date:
October 2024Approach:
Hybrid
Vaccine type:
R21/Matrix-M
Other malaria prevention tools deployed:
Seasonal malaria chemoprevention
 Gambia
Gambia
Implementation date:
To be determinedApproach:
To be determined
Vaccine type:
To be determined
Other malaria prevention tools deployed:
Seasonal malaria chemoprevention
 Ghana
Ghana
Implementation date:
May 2024Approach:
Age-based
Vaccine type:
RTS,S/AS01 & R21/Matrix-M
Other malaria prevention tools deployed:
Seasonal malaria chemoprevention
 Guinea-Bissau
Guinea-Bissau
Implementation date:
To be determinedApproach:
To be determined
Vaccine type:
To be determined
Other malaria prevention tools deployed:
Seasonal malaria chemoprevention
 Guinea
Guinea
Implementation date:
August 2025Approach:
Age-based
Vaccine type:
RTS,S/AS01
Other malaria prevention tools deployed:
Seasonal malaria chemoprevention, perennial malaria chemoprevention
 Ivory Coast
Ivory Coast
Implementation date:
July 2024Approach:
Age-based
Vaccine type:
R21/Matrix-M
Other malaria prevention tools deployed:
Seasonal malaria chemoprevention, perennial malaria chemoprevention
 Mali
Mali
Implementation date:
April 2025Approach:
Hybrid
Vaccine type:
R21/Matrix-M
Other malaria prevention tools deployed:
Seasonal malaria chemoprevention
 Niger
Niger
Implementation date:
September 2024Approach:
Age-based
Vaccine type:
To be determined
Other malaria prevention tools deployed:
Seasonal malaria chemoprevention
 Nigeria
Nigeria
Implementation date:
December 2024Approach:
Age-based
Vaccine type:
RTS,S/AS01
Other malaria prevention tools deployed:
Seasonal malaria chemoprevention, perennial malaria chemoprevention (pilot)
 Senegal
Senegal
Implementation date:
To be determinedApproach:
To be determined
Vaccine type:
To be determined
Other malaria prevention tools deployed:
Seasonal malaria chemoprevention
 Togo
Togo
Implementation date:
September 2025Approach:
Age-based
Vaccine type:
R21/Matrix-M
Other malaria prevention tools deployed:
Seasonal malaria chemoprevention, perennial malaria chemoprevention
The OPT-MVAC consortium’s workplan consists of five distinct work packages (WPs), each tasked with objectives and deliverables pertaining to the project and its execution.
Project management
Led by Michel Vaillant
WP1 will ensure the project’s objectives, milestones and deliverables are achieved and the workplan is implemented in compliance with the Grant Agreement and the Consortium Agreement. Responsibilities include project support for financial management, logistics, internal communications and coordination, in compliance with European Commission rules and procedures.
Scientific leadership, implementation research, training and research development
In collaboration with the Université of Thies, LSHTM and WHO-TDR – the Special Programme for Research and Training in Tropical Diseases, WP2 will provide technical support and training to help countries implement research protocols designed to monitor malaria vaccine introduction and identify barriers to uptake and develop mitigation strategies to address potential risks. WP2 will define project priorities and ensure compliance with good clinical practices and international standards.
Epidemiological methods, statistics and
data management
Led by Paul Milligan
WP3 will support reliable and timely data generation to drive improvements in immunisation performance. Activities will include harmonising monitoring and evaluation methods across countries to ensure data are comparable and supporting the ethical conduct of data collection, data integrity and data quality. Support to each country will be provided in-person and via email and WhatsApp, led by partners from LSHTM, with support from LIH, WHO-TDR, UIDT and experts in each country.
Safety and pharmacovigilance for
malaria vaccines
Led by Rachida Soulaymani
In collaboration with all consortium partners, WP4 will ensure the selection of administered vaccines in participating countries is based on a positive balance between benefits and risks; that pharmacovigilance systems have the capacity to collect and analyse data to proactively address risks; and that necessary resources are allocated to support the smooth pharmacovigilance operations.
Communications, dissemination and exploitation
Led by André-Marie Tchouatieu
WP5 will share information about the project, its progress and its findings with key stakeholders and engage in activities to facilitate improved access, coverage and trust in vaccines against all preventable infectious diseases
The OPT-MVAC consortium’s workplan consists of five distinct work packages (WPs), each tasked with objectives and deliverables pertaining to the project and its execution.







